The Science behind Ginger Milk Curd
Nothing beats the cold weather more than a warm bowl of ginger milk curd. This delicious dessert is very easy to prepare, but what intrigues me the most is the science behind it.
Recipe
Servings: 2 Preparation time: 30min
Ingredients
- Ginger juice 30g
- Milk 330g
- Milk powder 30g
- Sugar 12g
Equipments
Kitchen thermometer
Directions
- Pour 15g of ginger juice into each bowl.
- Dissolve milk powder and sugar into milk.
- Heat milk to 80 degree celsius.
- Pour milk quickly and evenly into each bowl.
- Rest for a few minutes, and observe the mixture solidifies.
Precautious
From my experience, it is better to use ceramic bowls instead of metal ones to prevent the milk from cooling too soon.
Science
I found little explanations online that aren’t too technical, so this is my attempt to explain the science.
Protein in Milk
80% of the proteins in cow’s milk is casein. It has 3 different types: $\alpha$-casein, $\beta$-casein and $\kappa$-casein. They don’t move around freely in milk, but grouped together to form casein micelle.
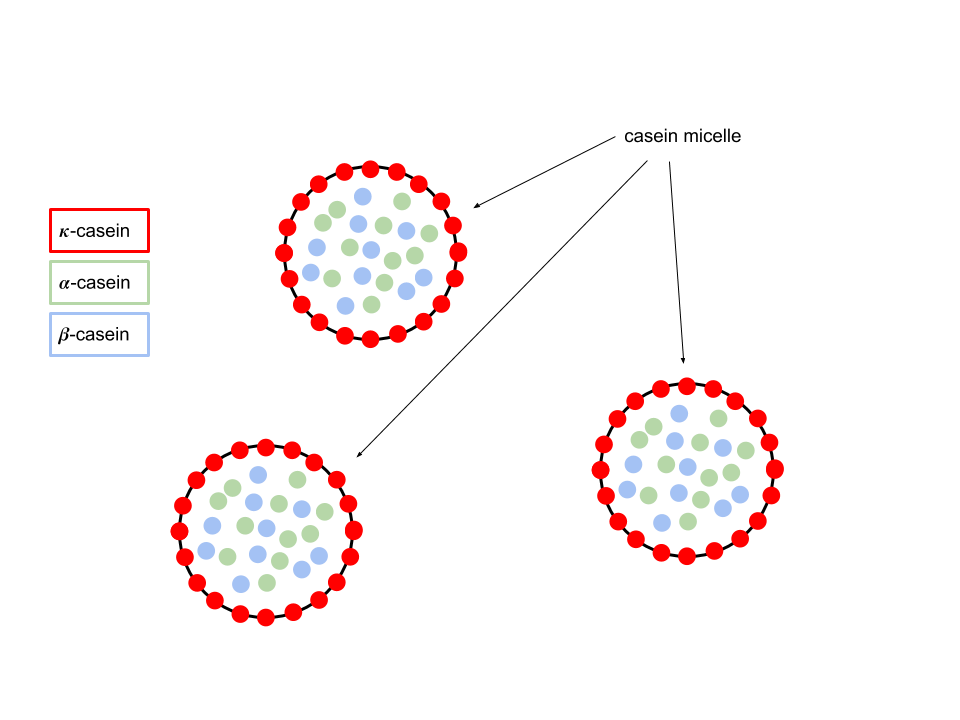
The outer layer of $\kappa$-casein is hydrophilic, which explains why it is stable in milk solution. On the other hand, both $\alpha$-casein and $\beta$-casein is hydrophobic.
Reacting with Ginger
Fresh ginger juice contains Zingibain protease, which can speed up the breakdown of proteins into smaller polypeptides.
When ginger juice is mixed with milk, zingibain cleaves on $\kappa$-casein to break it down into caseinomacropeptide (CMP) and para-kappa-casein. CMP is soluble and thus diffuses away from the micelle. What’s left is the para-kappa-casein that is hydrophobic. The hydrophobic parts of the micelles sticks to each other and thus milk curd firms.
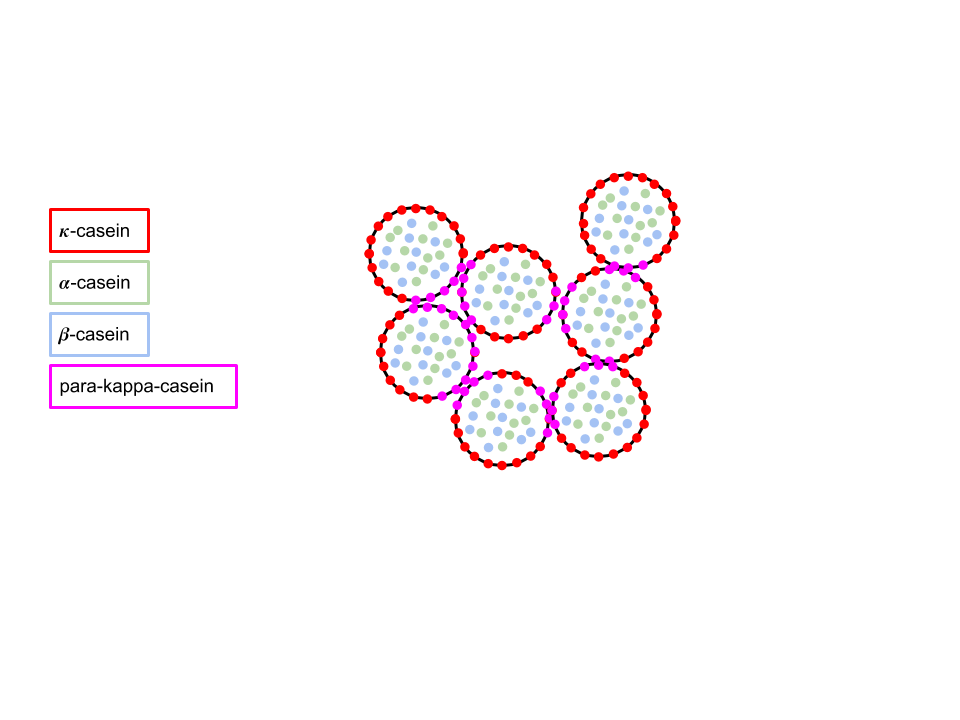
The temperature in which zingibain is most active is around 70 degree celsius, which explains why the recipe asks for milk of 80 degree celsius (accounts for the lowered temperature when milk is mixed with room temperature ginger juice). The high temperature also contributes to the hydrophobic reaction and speed up the forming of milk curds.
What can I do with this knowledge?
The best part of understanding the science behind a dish is that you can know whether other milk curd recipes will succeed before you even try it! For example, we know that soy milk couldn’t work because it lacks casein, or amount of sugar wouldn’t affect the result. I’ve gained many useful knowledge from a day worth of research and plans to continue doing so for other food. I hope it inspires you to explore too!
References
- 姜撞奶, 小高姐, https://youtu.be/QIqGeRnLGKk.
- What is Milk made of?, Nile Red, https://youtu.be/BV8aFRII1M4.
- Mechanism of acid and rennet casein coagulation, IGAI Food, https://youtu.be/ORy4yxrwQRY.
- Casein micelle aggregation, University of Guelph, https://www.uoguelph.ca/foodscience/node/1893/.
- Casein micelle structure, University of Guelph, https://www.uoguelph.ca/foodscience/book-page/structure-casein-micelle.
- 姜汁凝乳机理的探讨, 曾剑超, 2008.